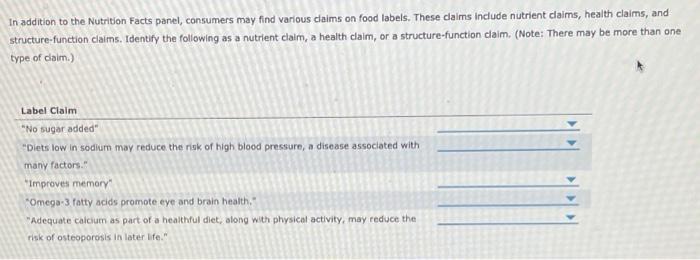
43 structure function claims on dietary supplement labels
› structurefunction-claimsStructure/Function Claims | FDA Mar 07, 2022 · Structure/Function Claims for dietary supplements ... appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 ... Structure/Function Claims Basics - Dietary Supplement Experts Structure/function claims describe the role of a nutrient or dietary ingredient intended to affect the structure or function of humans, or characterize the documented mechanism(s) of action by which a nutrient or dietary ingredient acts to maintain such structure or function. Importantly, they cannot be disease claims.
Should function claims be allowed on dietary supplements? Which claims can or Cannot be made on dietary supplement labels? Basically, dietary supplements cannot make 'disease' claims (for example: 'this supplement shrinks tumors'). Dietary supplements that make disease claims will be regulated by the FDA as drugs. Dietary supplements can make 'structure/function' claims (for example, 'calcium builds ...

Structure function claims on dietary supplement labels
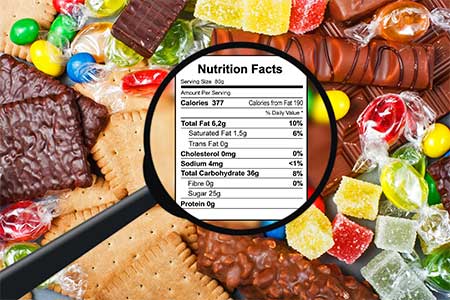
› food › dietary-supplements-guidanceDietary Supplement Labeling Guide: Chapter VI. Claims | FDA you must use a disclosure statement when you make a nutrient content claim and your food (including dietary supplements) contains one or more of the following nutrients in excess of the levels... Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Examples of structure/function claims include "Calcium builds strong bones" and "Antioxidants maintain cell integrity". Nutrient Content Claims Federal Preemption of 'Structure/Function' Claims on Dietary Supplements Federal Preemption of 'Structure/Function' Claims on Dietary Supplements. Federal law offers some protections to dietary supplement manufacturers against class actions claiming label statements ...
Structure function claims on dietary supplement labels. 10 Legal Tips to Stop FDA from Classifying Your Dietary Supplement as a ... For dietary supplements, FDA allows manufacturers and distributors to make 'structure/function claims' but does not allow 'disease claims.' A structure/function claim describes the role of a substance intended to maintain the structure of function of the body, or general well-being from consumption of a nutrient or dietary ingredient. › regulatory-information › search-fdaGuidance on Substantiation for Dietary Supplement Claims Please see the Federal Register of January 6, 2000 (65 FR 1000, codified at 21 CFR 101.93) for the final rule defining structure/function claims for dietary supplements and the January 9, 2002 ... Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,... FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in humans" or characterize the "documented mechanism" by which the nutrient acts to maintain such structure or function, but do not claim to "diagnose, …
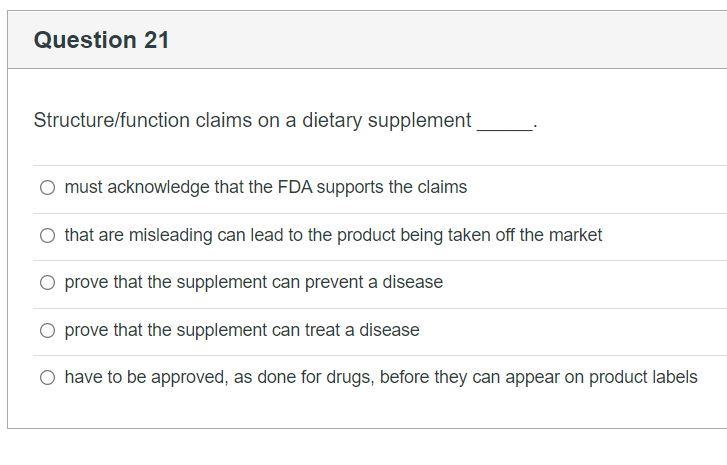
FDA Labeling Guidance: Making Structure/Function Claims for Dietary ... the fda permits supplements to so-called "structure-function claims," which are claims that describe the role of a nutrient or dietary supplement "intended to affect the structure or function in... Solved Question 21 Structure/function claims on a dietary - Chegg Transcribed image text: Question 21 Structure/function claims on a dietary supplement O must acknowledge that the FDA supports the claims that are misleading can lead to the product being taken off the market prove that the supplement can prevent a disease prove that the supplement can treat a disease O have to be approved, as done for drugs, before they can appear on product labels Dietary Supplements - Claims and Labeling Compliance for FDA/FTC Dietary supplements that make disease claims will be regulated by the FDA as drugs. Dietary supplements can make 'structure/function' claims (for example, 'calcium builds strong bones'). A structure/function claim describes the product's role in maintaining the 'structure or function of the body,' or 'general well-being.' Labeling rules are ... Solved In Week 2 you learned about Structure Function Claims | Chegg.com 1. Find an example of a Structure Function Claim on a label for a food or dietary supplement. Take a picture of the label, with the words of the structure function claim clearly visible. (if it takes 2 pictures, that's fine). Make sure you know what a Structure Function Claim is and that your example is a Structure Function Claim. 2.
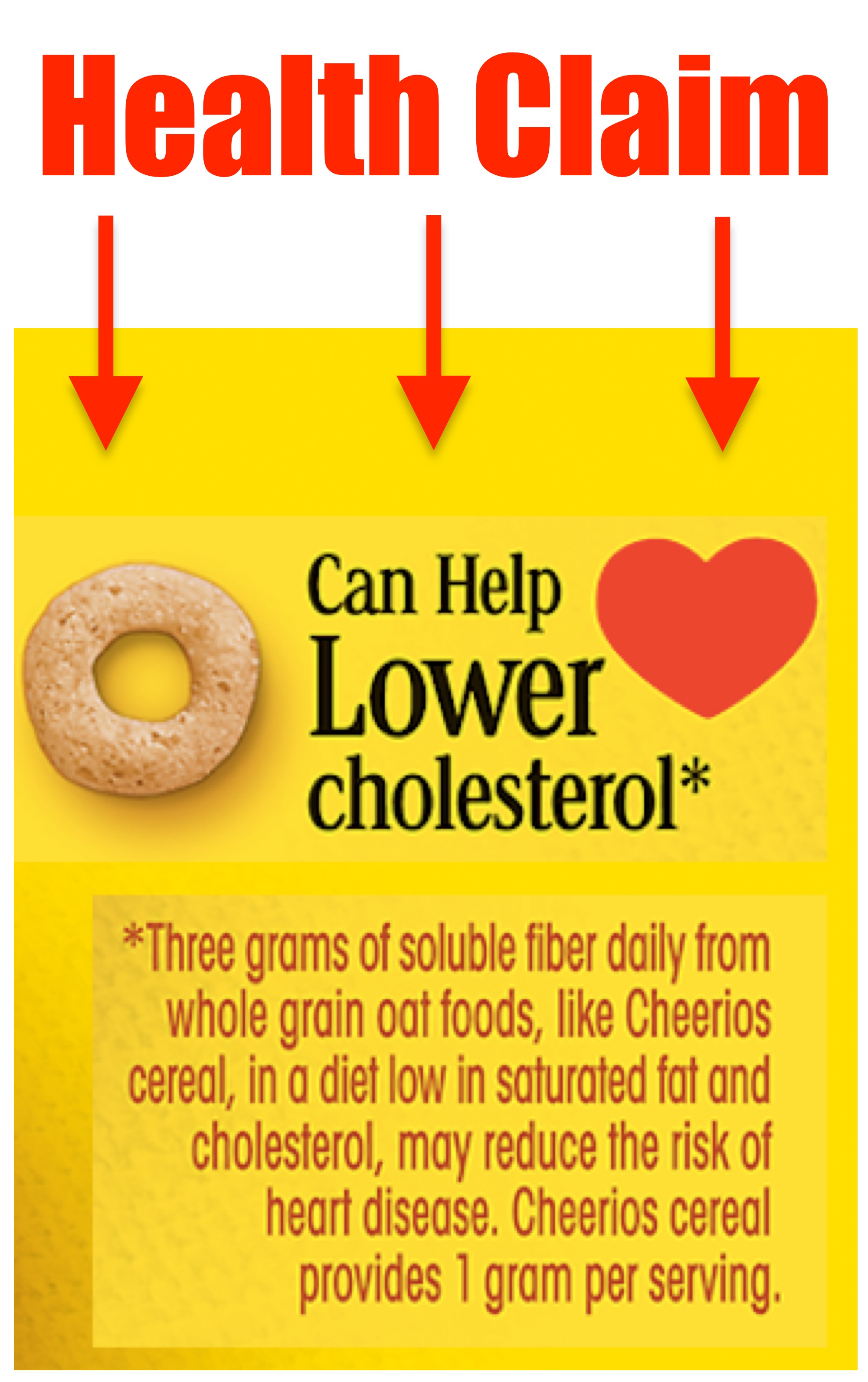
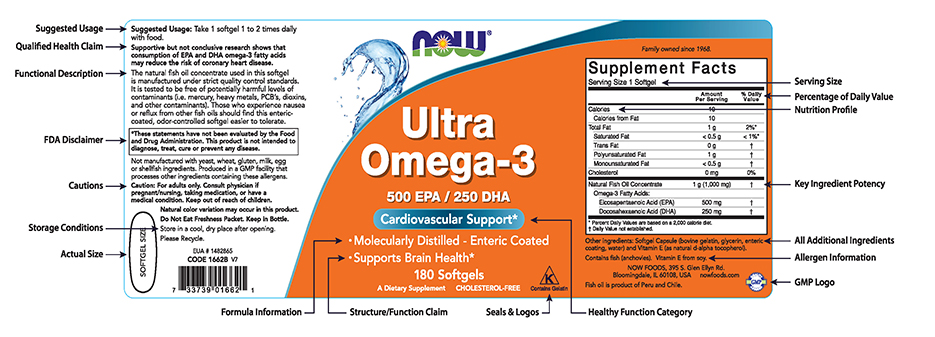
PDF Permissible vs. Impermissible Structure/Function Claims for Dietary ... Structure/Function Claims for Dietary Supplements. 2 THE BASICS: ... the heart symbol on product label and labeling is an impermissible heart disease prevention claim.) 14 ... No more than thirty (30) days after a supplement bearing a structure/function claim is marketed, the manufacturer, packer, or distributor of the Constructing structure and function claims - Natural Products INSIDER Structure/function claims describe a nutrient's or dietary ingredient's effect on or maintenance of the structure or function of the body—for example, "calcium builds strong bones" or "antioxidants maintain cell integrity." A disease claim is an express or implied claim to diagnose, mitigate, treat, cure or prevent a disease—for ... Dietary Supplements: An Advertising Guide for Industry Application of FTC Law to Dietary Supplement Advertising A. Identifying Claims and Interpreting Ad Meaning 1. Identifying Express and Implied Claims 2. When to Disclose Qualifying Information 3. Clear and Prominent Disclosure B. Substantiating Claims 1. Overview 2. Ads that Refer to a Specific Level of Support 3. The Amount and Type of Evidence 4. › regulatory-information › search-fdaSmall Entity Compliance Guide on Structure/Function Claims What are structure/function claims? The Dietary Supplement Health and Education Act of 1994 (DSHEA) added section 403(r)(6) ... Dietary supplement labels or labeling may, subject to the ...
Structure/Function Claim Notification for Dietary Supplements Office of Dietary Supplement Programs (HFS-810) Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive College Park, MD 20740-3835 Contact the Office of Dietary...
Structure Function Claims - Dietary Supplement Experts What laws and regulations govern structure/function claims? The Dietary Supplement Health and Education Act of 1994 (DSHEA) added section 403(r)(6) to the Federal Food, Drug, and Cosmetic Act (FD&C Act). This section of the law states that a dietary supplement may bear structure/function claims and provides their requirements.
Label Claims for Food and Dietary Supplement - LBS RCS.COM The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for structure/function claims and two related types of dietary supplement labeling claims, claims of general well-being and claims related to a nutrient deficiency disease.
''Structure/Function'' Claims in Dietary Supplement Labeling - ResearchGate Stephen H. McNamara Abstract Enactment of the Dietary Supplement Health and Education Act of 1994 (DSHEA) permitted manufacturers of dietary supplements to make structure/function' claims on...
› food › food-labeling-nutritionLabel Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for using structure/function claims and two related types of dietary ...
Federal Preemption of 'Structure/Function' Claims on Dietary Supplements Federal Preemption of 'Structure/Function' Claims on Dietary Supplements. Federal law offers some protections to dietary supplement manufacturers against class actions claiming label statements ...
Dietary Supplements Claims, Labels and Regulations | NSF Structure/function claims refer to the supplement's effect on the body's structure or function, including its overall effect on a person's well-being. Examples of structure/function claims include "Calcium builds strong bones" and "Antioxidants maintain cell integrity". Nutrient Content Claims
› food › dietary-supplements-guidanceDietary Supplement Labeling Guide: Chapter VI. Claims | FDA you must use a disclosure statement when you make a nutrient content claim and your food (including dietary supplements) contains one or more of the following nutrients in excess of the levels...
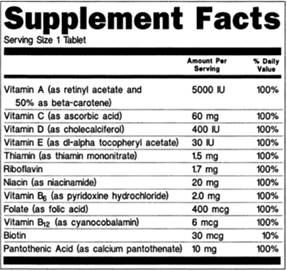




:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3652086/heart-check-label-crop.0.jpg)



:no_upscale()/cdn.vox-cdn.com/uploads/chorus_asset/file/3650624/quakerlabel-shelf.0.jpg)

















Post a Comment for "43 structure function claims on dietary supplement labels"